Martin Hoppstock

Martin Hoppstock
MD Student
Medical Doctor Project 13
MD13: Deciphering extracellular cold shock protein: immunoglobulin G complex formation and elucidating cellular effects thereof
|
|
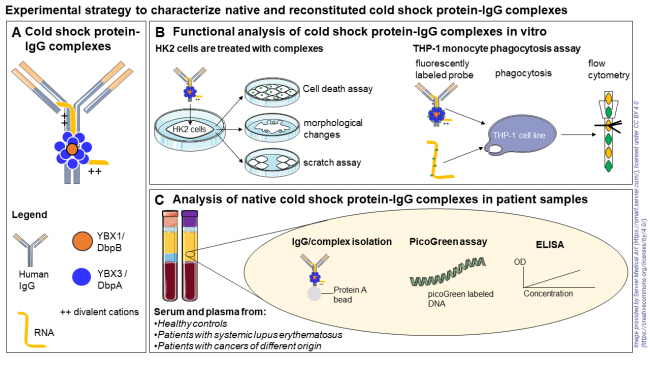
Cold shock proteins (CSPs) are among the most evolutionarily conserved protein families and are characterized by cold shock domains (CSDs) with highly conserved nucleic acid binding motifs. By interacting with single-stranded RNA and DNA, they regulate transcription, translation, and splicing, thereby influencing key processes such as cell proliferation. CSPs such as YB-1 and DbpA play central roles in stress responses and immune regulation acting extracellularly as alarmins, modulating immune activity, wound healing, and fibrogenesis. Our previous findings demonstrated that YB-1 and DbpA form high-molecular-weight complexes in serum and plasma, which co-migrate with immunoglobulin G (IgG). Depletion of IgG from human serum causes a marked reduction in CSP levels, confirming a physical interaction. This suggests the existence of protein complexes comprising IgG and CSPs, which can be selectively isolated from serum or plasma by IgG depletion. This project aims to investigate the cellular effects of native and reconstituted YB‑1/DbpA complexes. Using kidney cell models, we analyze how complex formation affects cytotoxicity, morphology, migration, and stress responses, including cytokine release. Patient studies will assess the abundance of YB-1/DbpA complexes in serum from individuals with systemic lupus erythematosus and cancer, and correlate levels with circulating DNA. Finally, uptake assays in monocytes will test whether these complexes facilitate DNA internalization and contribute to immune activation.
Panel A: Schematic representation of a reconstituted protein complex containing immunoglobulin G (IgG), cold shock proteins (YB-1 and DbpA), RNA, and divalent cations Panel B: Functional cell assays: Human kidney epithelial (HK2) cells are treated with reconstituted cold shock protein-IgG complexes to assess cytotoxicity (SYTOX and Annexin V assays), morphological changes (phase-contrast microscopy), cell migration (scratch assay), and stress or inflammatory signaling (cytokine and stress marker analysis). Panel C: Patient sample analysis: Native cold shock protein-IgG complexes are isolated from serum and plasma of healthy controls, patients with systemic lupus erythematosus, and patients with cancers of different origin using Protein A beads. Serum and plasma are analyzed by PicoGreen assay to quantify circulating cell-free DNA and by ELISA to measure YB-1/DbpA concentrations using a standard curve. |
Photos: by UMMD, Melitta Schubert/Sarah Kossmann