MD9: Direct and indirect intercellular communication between alveolar epithelium type 2 cells and T cells during chronic exposure to house dust mite allergens
|
|
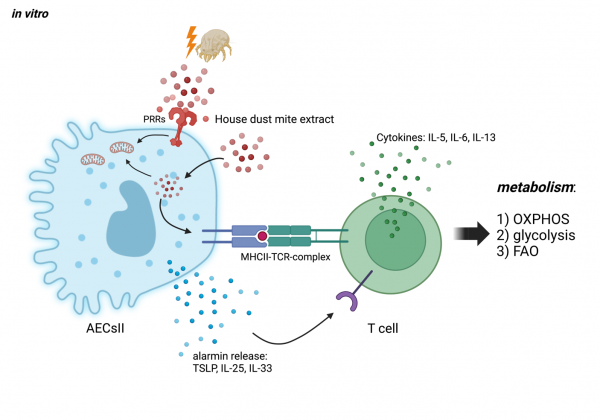
During allergic asthmatic airway inflammation, the epithelial barrier is significantly altered, which cumulates in maladaptation, chronic inflammation and the hallmark symptoms of allergic asthma such as shortness of breath, chest tightness and coughing. Sensitization and reexposure to allergens from house dust mites (HDM) cause alveolar epithelial type II cells (AECsII) to lose their barrier integrity and induce the release of soluble mediators, e.g., the alarmins TSLP, IL-33, and IL-25 that activate innate but also adaptive immune cells, including T helper (Th)2 cells. Furthermore, AECsII are able to express MHC-II molecules, which enables them to directly interact with T cell receptors (TCR) on T cells. In this project, we hypothesize that the direct and indirect intercellular communication between AECsII and Th2 cells affect the metabolism and intracellular signaling of both cell types and, thereby, the ability to create a disease promoting micromilieu that favors chronic allergic airway inflammation. Using an in vitro co-culture approach, we elucidate the distinct nature of the disease modifying interaction of AECsII and Th2 cells with the ultimate goal to understand how this contributes to maladaptive processes during allergic airway inflammation.
Interaction of AECsII and Th2 cells during exposure to house dust mite allergens. AECsII can internalize HDM by endocytosis, and present fragments of it via surface expressed MHC-II to TCRs of T cells. As a result, TCR signaling triggers alterations of important metabolic pathways in T cells like oxidative phosphorylation (OXPHOS), glycolysis and fatty acid oxidation (FAO). Furthermore, endotoxins are components of HDM that activate pattern recognition receptors (e. g. TLR4) on the surface of AECsII, which then release alarmins, including TSLP, IL-25 and IL-33 that can directly bind to corresponding receptors. |
Photos: by UMMD, Melitta Schubert/Sarah Kossmann