P4-3: Bidirectional cross-talk between Mast Cells and Endothelial Cells in homeostasis and inflammation
|
|
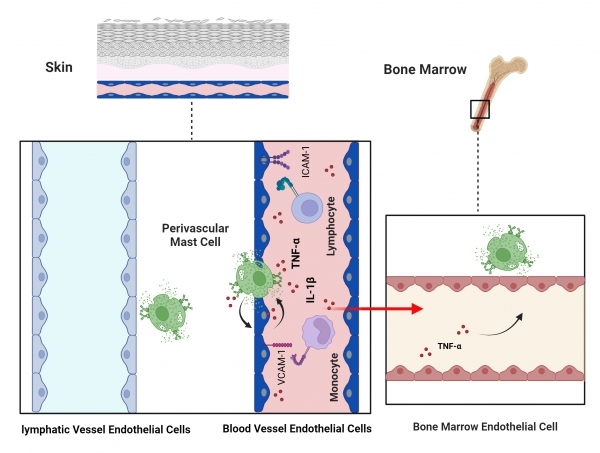
Mast cells (MCs) are tissue-resident innate immune cells that have critical functions in host defense, and play pivotal roles in allergic and inflammatory responses. They are ubiquitously distributed throughout the body, with a particular enrichment in barrier tissues, such as the skin, lungs, and the intestinal mucosa. Upon activation via different receptors, MCs release granules that are filled with a plethora of fully active mediators such as histamine, cytokines like TNF, and proteases. Since this process, called degranulation, occurs within seconds after their activation, MCs have important sentinel functions in the initiation of immune responses and thus as the first line of defense. We previously reported that, in the skin, MCs are located in very close proximity to blood vessels, and are critical for the early onset of innate immunity by rapidly initiating vascular responses and the recruitment of innate and adaptive immune effector cells. We hypothesize that in acute inflammation, MCs affect endothelial cell (EC) programming and functions, which may subsequently lead to dysregulation of the vasculature or long-term systemic effects on myeloid immune cell populations. This project aims to investigate the interactions between MCs and ECs during inflammatory responses on a tissue-wide, cellular and molecular level. The primary objective of this project is to explore how MCs influence EC characteristics and behavior, including their activation, expression of adhesion molecules, and transcriptional responses, both locally (in the skin) and systemically (e.g. in the bone marrow). We will employ a multifaceted experimental approach to uncover the mechanisms of MC-EC communication. Single-cell RNA sequencing (scRNAseq) of perivascular MCs will be utilized to identify key transcription factors and functional mediators involved in MC-EC crosstalk (cooperation with project 12-2, 12-3). Advanced multiscale imaging techniques, such as intravital microscopy, Lightsheet microscopy and multiparameter microscopy will allow for studying MC adhesion and activation dynamics in vivo and its mechanisms (cooperation with project 4-2). Complementing these studies, in vitro co-culture models with both human and mouse ECs and MCs will be developed to investigate direct cell-cell interactions and the impact of MC degranulation on EC activation and vascular integrity (cooperation with project 13-3).
|
Photos: by UMMD, Melitta Schubert/Sarah Kossmann