Dr. Ilka Müller

Dr. Ilka Müller
Former Associated Postdoc
Associated Project 6
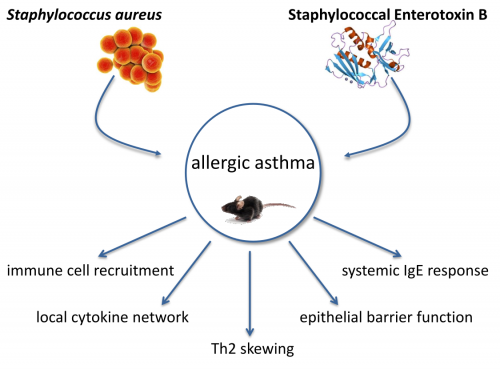
AP6: Effects of respiratory infections and microbial carriage on the induction and shape of allergic asthma
|
|
Over the last decades the incidence of allergic diseases such as allergic asthma was on the rise in western industrial countries. By now there are over 300 million asthmatics worldwide. Asthma is a heterogeneous chronic respiratory disease and about 60 % of adult asthmatics suffer from allergic asthma, which is mainly Th2‑driven. Patients suffer from airway hyperreactivity, variable bronchial obstruction, increased mucus production and structural changes of the airways. It has been proposed that allergic sensitization and inflammation in asthma can be affected by Staphylococcus aureus (S. aureus). S. aureus is a gram-positive bacterium and facultative pathogen which colonizes up to 30 % of the adult population. In newborns the colonization rate is even higher as in 70 % of all newborns S. aureus could be detected. The anterior nares of the nose are the most frequent carriage site of S. aureus. Beside the upper airways S. aureus colonizes the skin, the pharynx as well as the gastrointestinal tract. The described associations with allergic asthma are of high medical relevance but are not well understood to date. The project aims at elucidating the immunological mechanisms underlying the association between S. aureus and allergic asthma in relevant mouse models. One major focus is the immune regulatory potential of Staphylococcal enterotoxin B (SEB) in allergic asthma. SEB is a small protein with a molecular weight of 28.4 kDa, one of the major superantigens produced by S. aureus and a potent activator of the immune system. The effects of an intranasal treatment with SEB alone or additional SEB-treatment during different phases of allergic asthma in a mouse model will be analyzed regarding histological changes, respiratory and systemic inflammation as well as IgE responses. The local inflammation, assessing immune cell populations and their activation status will be characterized. Furthermore concentrations of different cytokines and chemokines will be analyzed, as well as OVA‑specific and SEB‑specific IgE-levels. Additionally, detailed histological examinations will be performed and airway hyperreactivity will be assessed in order to address SEB-mediated effects on airway hyperreactivity in the asthma mouse-model. Taken together, these detailed analyses of the immunological mediators, recruited immune cells, IgE levels and lung function parameters in SEB‑treated, asthmatic, SEB-treated asthmatic and control mice will yield a comprehensive insight into SEB-mediated immune modulation as well as into its effects on allergic asthma. Importantly, these analyses will build a solid basis for future projects such as pre-clinical interventional studies. Here, the contribution of key mediators could be analyzed e.g. through neutralization via specific antibodies or using knock-out mouse models.
|
Photos: by UMMD, Melitta Schubert/Sarah Kossmann