Dr. Shruthi Krishnan

Dr. Shruthi Krishnan
Former PhD Student/Postdoc
Start-up Funding Project 1, Project 9-1
Start-up Funding
SF1: Role of CD248 in mTORC1-mediated unfolded protein response (UPR) in diabetic kidney disease
|
|
Initial hypothesis and aims of the project Based on previously published data and our preliminary data, we hypothesize that CD248 promotes diabetic kidney disease (DKD) by inhibiting the adaptive UPR response, but also by promoting maladaptive signaling. Specifically, we hypothesize that CD248 enhances ATF4 and ATF6α signaling via the UPR, inducing the maladaptive transcription factor CHOP and inflammation by modulating NF-κB signaling. Based on preliminary data and the function of UPR- and mTORC1-signalling in DKD, we also hypothesize that CD248-mediated mTORC1 pathway regulates UPR signaling, thus contributing to renal inflammation and cell death in DKD. Aims of the project:
|
Photos: by UMMD, Melitta Schubert/Sarah Kossmann
P9-1: Unfolded protein response (UPR) in diabetic kideny disease and H. pylori infected gastric mucosa
Shruthi KrishnanPhD Student Yanfei YuPhD Student
Berend IsermannProject Leader
Michael NaumannProject Leader |
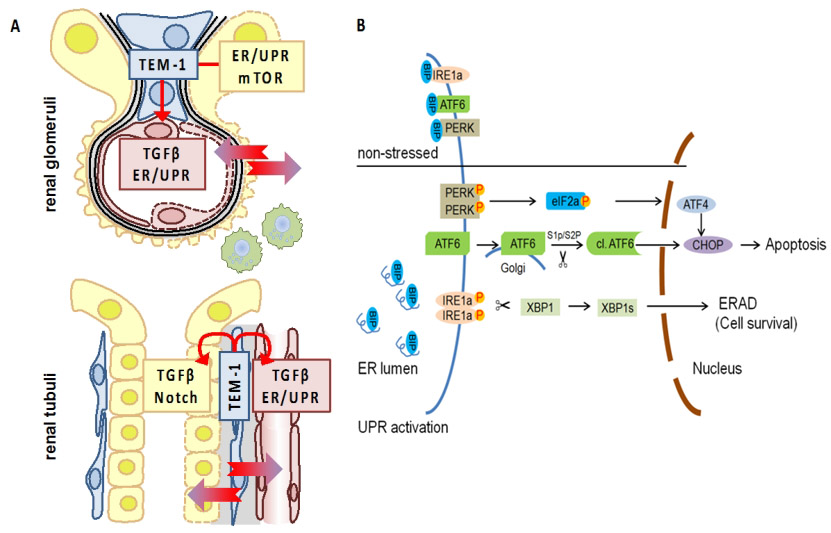
Diabetic nephropathy (dNP), now the most common cause of chronic renal disease, is characterized by dysfunctional barriers in the glomerular and tubular compartments. A common cell type found in both renal compartments are pericytes, which interact with epithelial (EpiC) and endothelial (EndoC) cells. Maladaptive pericyte activation impairs barrier function and promotes kidney fibrosis, a common final pathway in kidney damage. Targeting pericyte function may allow protection of both renal compartments. However, specific strategies to target pericytes are currently lacking. TEM1 (tumor endothelial marker 1, CD248, or endosialin) is a protein expressed by pericytes in various organs, including the kidney and the stomach. Expression of TEM1 is high during development in tumors and during inflammatory diseases. In a clinical study low TEM1 expression by cancer associated fibroblast correlates with increased survival of gastric cancer patients. However, the mechanisms underlying TEM1-dependent effects remain poorly defined. In cooperative work (Project 7) we analyzed TEM1 regulation and the unfolded protein response (UPR) and established a role of TEM1 in renal pericytes in the context of dNP. We hypothesize that pericyte derived TEM1 co-ordinately regulates barrier function in chronic diseases. Specifically, we define the molecular mechanism and functional consequences of intracellular signaling via TEM1 between pericytes and barrier-defining epithelial cells, and conduct a structure function analyses of TEM1 to identify the molecular structures required for barrier regulation to determine whether modulating TEM1 function allows to rescue epithelial cells and thus barrier function. Two PhD students jointly address questions about the regulation of the UPR in the context of renal cells (Shruthi Krishnan) and H. pylori-infected gastric mucosal cells (Yanfei Yu).
UPR regulation in DKD and H. pylori infection. (A) TEM-1 expressing cells in the kidney (blue; mesangial cells, top, and peritubular pericytes, bottom) interact with endothelial cells (red), podocytes (yellow, top), and tubular cells (yellow, bottom). (B) Schematic diagram of UPR signalling. |
Photos: by UMMD, Melitta Schubert/Sarah Kossmann