Prof. Dr. Berend Isermann

Prof. Dr. Berend Isermann
Former Project Leader (10/2018 - 09/2021)
Project 7, Project 9
P7-1: Maladaptive cross-talk at the glomerular filtration barrier
Akash MathewPhD Student Berend IsermannProject Leader |
Diabetic nephropathy (dNP) is the leading cause of renal failure in industrialized countries. Beyond inhibition of the angiotensin-system efficient therapies for dNP are lacking. While the relevance of individual cell components at the glomerular filtration barrier is well-studied, the mechanisms through which these cells coordinately regulate glomerular homeostasis and function remain largely unknown. Persistent hyperglycaemia promotes cellular dysfunction through maladaptive mitochondrial and ER-signalling. This is associated with altered PTMs of important homeostatic regulators (e.g. NF-κB, HIF, sXBP-1, HSPs). These maladaptive responses are molecular fixed and perpetuated through PTM’s and epigenetic reprogramming. We previously demonstrated that cellular interactions and protease-dependent signaling at the glomerular filtration barrier determine the highly specialized glomerular cell phenotype and epigenetically control expression of the redox-regulator p66Shc. These interactions are disrupted in a hyperglycaemic milieu. How cells at the glomerular filtration barrier interact during the maladaptive process, resulting in a specific disease promoting micromilieu, remains unknown. We will use an innovative co-culture system mimicking the glomerular filtration barrier to conduct kinetic studies aiming to identify extracellular mediators (e.g. coagulation proteases, YB-1, syndecan-4) and PTMs determining the cellular phenotype and function (cooperation with Project 6 and Project 8). This in vitro model will enable us to include tubular cells. These studies will be supplemented with expression profiling and ChIP sequencing (e.g. NF-κB, HIF, sXBP-1; cooperation with Project 6). Kinetic in vivo imaging analyses using genetically designed mice (loss or gain of function in the proteostasis network) will be used to ascertain the mechanistic relevance of identified regulators in vivo (cooperation with Project 4). Findings will be validated using in vitro (loss-/gain-of-function) and in vivo (tissue-specific gene-modulation, tubular-, podocyte-, or endothelial-specific Cre-expression) approaches as well as ex vivo analyses of human tissue biopsies.
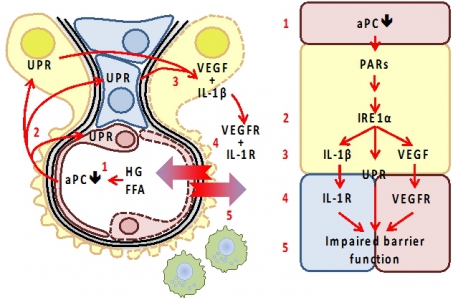
Maladaptive intercellular cross-talk at the GFB impairs barrier function. Elevated glucose (high glucose, HG) and free fatty acid (FFA) concentrations cause endothelial dysfunction, impairing EndoC-dependent protein C activation (1). Activated protein C (aPC) acts on endothelial cells (red), but also on podocytes (epithelial cells; yellow) and mesangial cells (blue), conveying cytoprotective effects via protease activated receptors. Loss aPC induces the unfolded protein response (UPR, 2) by directly or indirectly (via p66Shc) modulating ER-stress signaling (IRE1α). We speculate that IRE1α orchestrates UPR, inflammasome activity (IL-1β), and VEGF release (3). The corresponding mediators co-ordinately alter GFB function through receptor dependent mechanisms (4, 5). Excess IL-1β, VEGF, or yet to be identified mediators impair the barrier function. |
Photos: by UMMD, Melitta Schubert/Sarah Kossmann
P9-1: Unfolded protein response (UPR) in diabetic kideny disease and H. pylori infected gastric mucosa
Shruthi KrishnanPhD Student Yanfei YuPhD Student
Berend IsermannProject Leader
Michael NaumannProject Leader |
Diabetic nephropathy (dNP), now the most common cause of chronic renal disease, is characterized by dysfunctional barriers in the glomerular and tubular compartments. A common cell type found in both renal compartments are pericytes, which interact with epithelial (EpiC) and endothelial (EndoC) cells. Maladaptive pericyte activation impairs barrier function and promotes kidney fibrosis, a common final pathway in kidney damage. Targeting pericyte function may allow protection of both renal compartments. However, specific strategies to target pericytes are currently lacking. TEM1 (tumor endothelial marker 1, CD248, or endosialin) is a protein expressed by pericytes in various organs, including the kidney and the stomach. Expression of TEM1 is high during development in tumors and during inflammatory diseases. In a clinical study low TEM1 expression by cancer associated fibroblast correlates with increased survival of gastric cancer patients. However, the mechanisms underlying TEM1-dependent effects remain poorly defined. In cooperative work (Project 7) we analyzed TEM1 regulation and the unfolded protein response (UPR) and established a role of TEM1 in renal pericytes in the context of dNP. We hypothesize that pericyte derived TEM1 co-ordinately regulates barrier function in chronic diseases. Specifically, we define the molecular mechanism and functional consequences of intracellular signaling via TEM1 between pericytes and barrier-defining epithelial cells, and conduct a structure function analyses of TEM1 to identify the molecular structures required for barrier regulation to determine whether modulating TEM1 function allows to rescue epithelial cells and thus barrier function. Two PhD students jointly address questions about the regulation of the UPR in the context of renal cells (Shruthi Krishnan) and H. pylori-infected gastric mucosal cells (Yanfei Yu).
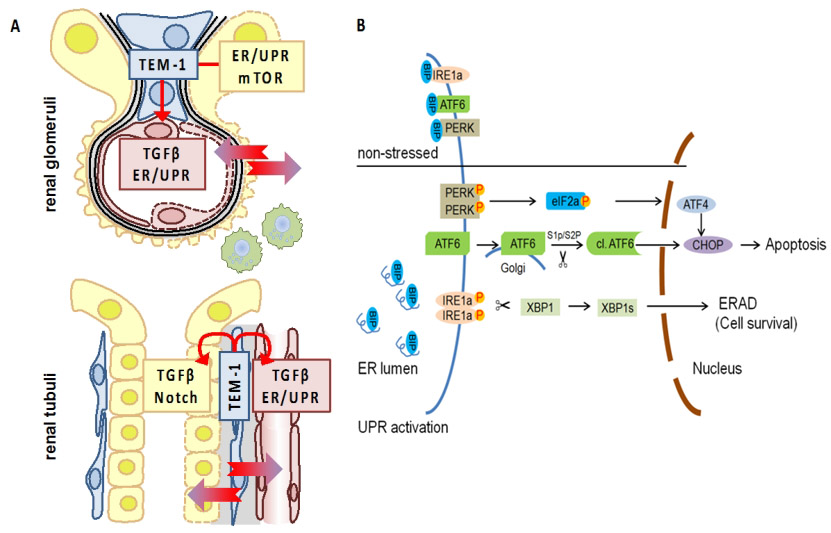
UPR regulation in DKD and H. pylori infection. (A) TEM-1 expressing cells in the kidney (blue; mesangial cells, top, and peritubular pericytes, bottom) interact with endothelial cells (red), podocytes (yellow, top), and tubular cells (yellow, bottom). (B) Schematic diagram of UPR signalling. |
Photos: by UMMD, Melitta Schubert/Sarah Kossmann
AP4: Role of platelets and transcription factor p45-NFE2 for renal barrier function in diabetic nephropathy
|
|
Diabetic nephropathy (dNP) is a chronic kidney disease observed in patients with diabetes mellitus (DM). An altered hemostatic system and an important role of coagulation proteases in the pathophysiology of dNP is well established. Patients with DM have platelet-hyperreactivity and enhanced platelet activation. However, studies evaluating a mechanistic role of platelets in dNP are lacking. In this regard, when activated platelets come in contact with the injured glomerular filtration barrier, it can result in glomerular endothelial injury and production of pro-inflammatory cytokines. This results in glomerular endothelial dysfunction and loss of barrier function. Loss of NF-E2, which is required for the completion of megakaryocyte maturation and platelet production, results in a quantitative platelet deficiency in mice, making it a suitable model to study the role of platelets in diseases. Interestingly, a platelet independent and trophoblast specific role of NF-E2 for placental development has been shown. Mechanistically, these placental effects are regulated through epigenetic modifications such as acetylation and sumoylation. However, the role of platelet dependent as well platelet independent (renal resident cell dependent) role of p45 NF-E2 and associated mechanisms insights in dNP remains unknown. Therefore, within the current study we will dissect the role of platelets as well as transcription factor p45-NFE2 in dNP. In order to specifically address the role of platelets, we will inhibit platelet activation using aspirin (pharmaceutical inhibition) or using platelet specific Gaq knockout mice (GaqLoxP x PF4cre, genetic inhibition). We will further study the effect of platelets on glomerular endothelial barrier function by evaluating markers of endothelial dysfunction (eNOS, tight junction proteins). Markers for cell death (TUNEL) and inflammation (IL 1β and NLRP3) will be evaluated using immunoblotting and staining in glomerular endothelial cells. Furthermore, we will perform trans-endothelial barrier assays using microfluidics based approach on co-cultures of knockout GENC and podocytes. To study the role of p45 NFE-2 in renal resident cells, we will first conduct in vitro studies to identify the renal cell types which express NFE2 and are relevant for our model. We will then study the effect of high glucose in NFE2 deficient cells which will be generated using CRISPR or shRNA knockdown approaches. Mechanistically, we will study molecular markers of inflammation (IL 1β, HMGB1), kidney injury (KIM-1, Nephrin), cell death (TUNEL) and senescence (p21, p16, β-Gal) using immunoblotting and staining. Furthermore, we will use NFE2LoxP mice to delete the expression of NFE2 in relevant renal resident cells or platelets. Mechanisms of cellular proliferation, senescence and epigenetic modifications will be evaluated in NFE-2 deficient cells in cooperation with Project 5 and Project 7. Taken together, these studies will establish the contribution of platelets in the pathogenesis of dNP and distinguish it from the role of p45 NF-E2 in renal resident cells.
Disturbances in the endothelial function and coagulation pathway may cause initial platelet activation. Intrinsic changes in the platelet metabolism and changes in intra-platelet signaling pathways due to diabetes contribute to the increased platelet hyperactivity in T2DM. This results in glomerular endothelial dysfunction which is characterized by loss of eNOS and fenestrations. Danger signals released from platelets can activate inflammasome and promote cell death disrupting the barrier. On the other hand, a renal cell specific loss of NFE2, a transcription factor regulating platelet formation, can result in impaired cellular proliferation and kidney regeneration. Furthermore, cell intrinsic NFE2 can modulate renal cell metabolism by regulating epigenetic modifications. |
Photos: by UMMD, Melitta Schubert/Sarah Kossmann