Prof. Dr. Rüdiger Braun-Dullaeus

Prof. Dr. Rüdiger Braun-Dullaeus
Former Project Leader (10/2018 - 03/2023)
Project 6
P6-1: Normoxic HIF stabilization at the vascular barrier in atherosclerosis
Mohsen Abdi
|
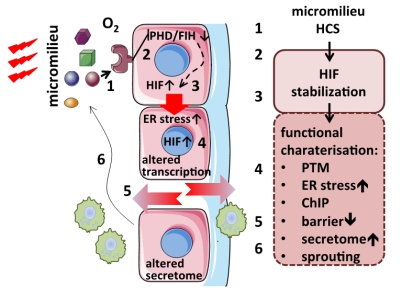
Pathophysiologically, atherosclerosis is characterized by chronic inflammation and one of its early hallmarks is activation of the endothelium. In response to endothelial injury, locally released mediators induce a maladaptive endothelial process, which perpetuates inflammation and proliferation, thus promoting plaque growth. Beside the activation of classical pro-inflammatory signaling pathways, i.e. NFκB, PTMs and stabilization of the transcription factor family of hypoxia inducible factors (HIFs) has been demonstrated, even in the absence of hypoxia at the endothelial barrier or within the atherosclerotic plaque. Various factors causing normoxic HIF stabilization have been identified. However, a systematic approach is missing. HIFs are known to impair proteostasis (autophagy, UPR) and to transactivate the transcription of genes of glycolysis, growth factors and matrix metalloproteinases, thus generating a disease promoting micromilieu. We hypothesize that normoxic stabilization and activation of HIFs at the vascular barrier lead to a disease promoting vicious circle of inflammation and impaired proteostasis. We will conduct a systematic approach to analyze HIF stabilizing capabilities of various growth factors, pro-inflammatory cytokines, and factors of cellular senescence in a time and dose dependent manner utilizing life cell imaging of a far-red fluorescent variant of HIF1α in endothelial cells and subsequent high content image analysis (cooperation with Project 1). Stabilizing agents will be further characterized with regard to HIF-dependent gene-expression (expression profiling, ChIP sequencing; cooperation with Project 9), proteostasis (UPR, autophagy; cooperation with Project 5 and Project 7), and PTM (cooperation with Project 7 and Project 8) in a time-dependent fashion. Co-cultures (endothelial cells, smooth muscle cells, macrophages) and microfluidic systems will be used to conduct loss- and gain-of-function experiments (cooperation with Project 1). Results will then be cross-validated in a translational approach by analysis of human atherosclerotic plaque tissues. These comprehensive studies will determine regulation and pathophysiological relevance of normoxic stabilization of HIFs at the endothelial barrier and its impact on the pro-atherogenic micromilieu.
Normoxic stabilization of HIFs and functional consequences. The initial high content screen (HCS) will reveal HIF-stabilizing micromilieu factors (1). Underlying signaling pathways will be assessed using inhibitors (2). Functional consequences of HIF-stabilization (3) will be characterized with regard to PTM (4), ER stress, barrier function (5), and leucocyte transmigration. ChIP sequencing will be used to identify normoxic specific HIF target genes. (Patho-) physiological implications will be evaluated by analyzing barrier function and the secretome and by conducting sprouting and tube formation assays (6). |
Photos: by UMMD, Melitta Schubert/Sarah Kossmann
CS2: The role of VAMP3-IKKg/NEMO interaction in inflammation of atherosclerosis
|
|
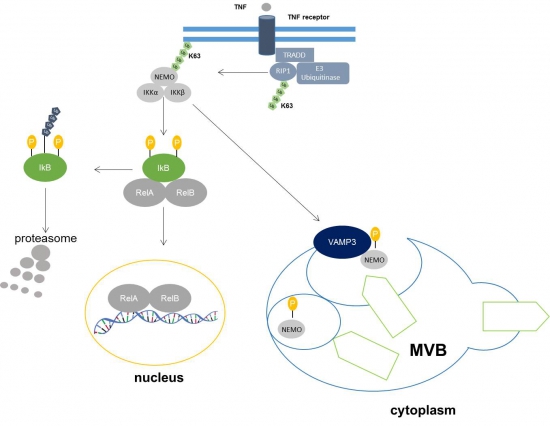
Atherosclerosis is the cause of vascular diseases like myocardial infarction and stroke. Cardiovascular diseases are the leading cause of mortality worldwide. Atherosclerosis comprises an inflammatory micromilieu which results in an impaired barrier function. This triggers the retention of Apolipoprotein B (apoB), which provokes an entry of monocytes and their differentiation into macrophages. One of the key steps in early atherosclerotic inflammation is the activation of NF-kappaB (NF-κB) signaling pathway in endothelial cells and macrophages. Essential for activation of the NF-κB signaling pathway is the IKK complex, which phosphorylates the inhibitor of NF-κB transcription factor, IΚB. Subsequently, IKB is proteasomally degraded allowing the NF-κB transcription factor to translocate into the nucleus. The IKK complex consists of IKKα and IKKβ as catalytical subunits, and the NF-κB essential modifier (IKKγ/NEMO). VAMP3 is a SNARE (soluble N-ethylmaleimide-sensitive-factor-attachment receptor) protein involved e.g. in endocytic and exocytic pathways. It is necessary for the fusion between autophagosomes and MVBs (Multivesicular Bodies) and interacts with NEMO. Hitherto we lack insights how the interaction of VAMP3 and IKKγ/NEMO modulates NF-κB signaling.
|
Photos: by UMMD, Melitta Schubert/Sarah Kossmann