P5-1: Maladaptive unfolded protein response (UPR) and senescence at the vascular barrier in diabetes mellitus
Sameen FatimaPhD Student Khurrum ShahzadProject Leader |
Among the major risk factors for cardiovascular disease is the presence of diabetes mellitus (DM). The disease process differs between non-diabetic and diabetic individuals, as exemplified by an accelerated disease course and different plaque morphology, plaque stability, and inflammatory cell composition independent of cholesterol levels and age. The mechanisms resulting in the different atherosclerotic disease course in diabetic and non-diabetic patients remain largely unknown. We speculate that the hyperglycaemic milieu aggravates maladaptive processes at the vascular barrier and that hyperglycaemia induced unfolded protein response (UPR) and PTMs cause molecular fixation (e.g. epigenetic changes) and hence perpetuation of the disease process. Based on unpublished data we hypothesize that these changes induce senescence (including the senescence associated secretory phenotype, SASP), resulting in a disease promoting micromilieu within the plaque. To determine the functional relevance of senescence we will specifically deplete p16-expressing cells in vivo (conditional p16-driven DTR-expression, Cadh5Cre, αSMCCre, LysMCre). The UPR will be analyzed in vivo using mice with cell-specific gain- or loss-of-function mutations of key UPR-regulators (ATF6, sXBP1; cooperation with Project 7). Lesion development and phenotype (Oil-red-O stain, Movat stain), plaque composition (immunohistochemistry, laser dissection), senescence (β-galactosidase, p27), UPR (e.g. ATF6, sXBP1, ChOP), and ROS (e.g. ONOO, 8-OH-dG) will be analyzed ex vivo using tissue samples from control, hyperglycaemic, and hyperlipidaemic ApoE-/- mice and patients samples (cooperation with Project 2, Project 6, and Project 7). These in vivo approaches will be paired by detailed in vitro experiments mimicking the vascular barrier (modified Bowden chamber, microfluidics; cooperation with Project 2). The ex vivo models will allow detailed mechanistic studies using cell-specific gain and loss of function approaches and large scale approaches to characterize in a temporal fashion gene-expression and epigenetic gene-regulation (e.g. ChIP sequencing for sXBP1, ATF6, ATF4; cooperation with Project 7 and Project 9).
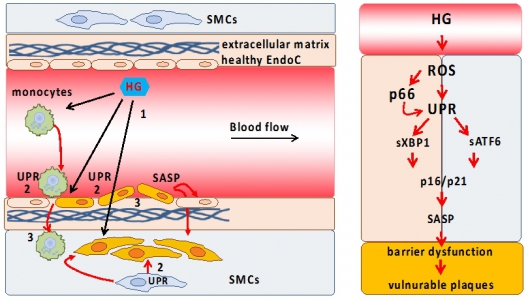
Cellular senescence as cause of endothelial barrier dysfunction in atherosclerosis. Schematic representation of proposed vascular barrier dysfunction (left) and pathological signaling pathways involved (right). High glucose (HG, 1) induces an unfolded protein response (UPR, 2) and senescence in barrier defining cells, disrupting the barrier (3); ROS (reactive oxygen species); SASP (senescence-associated secretory phenotype); EndoC (endothelial cells, red); SMC (smooth muscle cells, blue); orange color represents senescent cells. |
Photos: by UMMD, Melitta Schubert/Sarah Kossmann