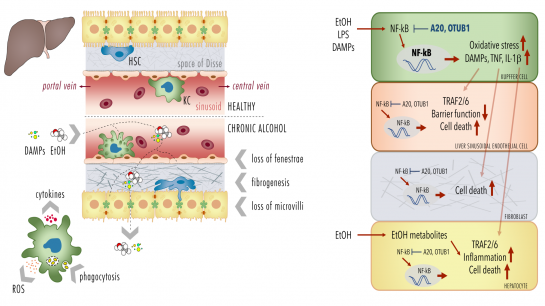
P10-1: Maladaptation of the hepatic barrier in alcohol-induced liver injury
Aleksander NowakPhD Student
Borna ReljaProject Leader |
Alcoholic liver disease (ALD) as one of the predominant causes of liver-related morbidity and mortality worldwide encompasses a spectrum of liver injury ranging from simple steatosis to steatohepatitis, fibrosis, and finally cirrhosis. The pathogenesis of this multifactorial disease in-volves both hepatic non-parenchymal and parenchymal cells (hepatocytes). The project focus on functional studies in a murine model and isolated primary Kupffer cells (KCs), liver sinusoidal endothelial cells (LSECs) and hepatocytes from mice being chronically fed with a Lieber-DeCarli diet containing alcohol (ethanol, EtOH) or an isocaloric control diet. Following induction of the early stage of ALD, comparative analyses will be conducted in the murine model, scrutinizing hepatic barrier integrity and systemic and local inflammation. Herein chemokines, cytokines, DAMPs, leukocyte activation and hepatic infiltration via immuno-histology, flow cytometry, organ histopathology will be analysed (cooperation with Project 7, Project 8 and Project 9). Further, loss of fenestrae, fibrogenesis, necroptosis, apoptosis, pyroptosis, and oxidative burst as well as phagocytosis by KCs in different cell types will be investigated. In addition, the NF-κB activity and cellular responses (cytokine release, cell survival) of each isolated primary cell type (KCs, LSECs and hepato-cytes) will be studied (cooperation with Project 1). Cells will be isolated by enzymatic digestion of liver tissue and gradient centrifugation. For the isolation of cells selective adherence behaviour (KCs), and subsequent F4/80 (KCs), CD45 and CD31 (LSECs) or ASGPR (hepatocytes) will be used as signature expression markers. NF-κB signaling is regulated by a variety of posttranscriptional modifications (PTMs), including covalent conjugated ubiquitin. Deubiquitinylating enzymes (DUB) cleave ubiquitin from substrate proteins and are hence key regulators of the NF-κB sys-tem. DUBs A20 or OTUB1 regulate/terminate TNF- or IL-1β-induced NF-κB activation, respec-tively, suppressing inflammation and oxidative stress, but also DNA repair and cell death. To determine the causality of DUBs, selected DUBs will be knocked down (A20 and OTUB1) and the consequences of chronic exposure to EtOH, or stimulation with endotoxin or DAMPs on NF-κB activity, cytokine release, inflammasome activation and cell survival (immunoblots, ELISA, FACS) will be evaluated in isolated primary cells (KCs, LSECs and hepatocytes) and different hepatic human cell lines (human Kupffer cells, HLSEC/ciJ LSECs, HepG2 and AML12 hepato-cytes etc. in cooperation with Project 1 and Project 7).
Proposed mechanisms of A20 and OTUB1-mediated maladaptation of the hepatic barrier loss. |
Photos: by UMMD, Melitta Schubert/Sarah Kossmann