CS3: The role of the cold shock protein YB-1 and its autoantibodies in the disease process of Alzheimer's disease
|
|
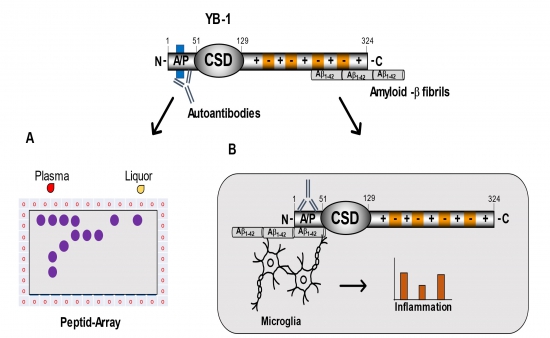
The most common neurodegenerative disease-causing dementia is Alzheimer's disease. Current understanding of pathophysiology links progressive memory loss with neuronal cell death due to extracellular amyloid plaque formation and intracellular deposition of neurofibrillary tangles. Despite intensive research, the disease remains an enigma, although there is substantial evidence that both proinflammatory cytokines and autoantibodies not only play a minor role but can also be linked to causal factors. YB-1 is the prototypical member of cold shock proteins in humans and fulfills pleiotropic functions in the cell cycle, differentiation, stress response, DNA repair and inflammation response. In an Alzheimer's animal model, nasal immunization with YB-1 resulted in amyloid fibril destruction and cognitive improvement. Also, YB-1 could be linked within the process of senescence. In our own preliminary work, specific YB-1 autoantibody epitopes have already been disclosed in tumor patients. In addition, preliminary examinations in patients with Alzheimer's disease show another specific autoantibody epitope. The research project aims to investigate the hypothesis of whether the specific YB-1 autoantibody epitope (i) is established as a diagnostic marker in a large cohort of Alzheimer's patients or (ii) can be used as an early diagnostic marker (MCI, SCI), whether (iii) Autoantibodies play a role in the YB-1 biology and (iv) if they can influence the neuroinflammatory process. In this context, patients are recruited with the DZNE. Then experiments in protein biochemistry and cell culture are carried out. Plasma samples from Alzheimer's patients show a specific epitope in the N-terminal section of the cold shock protein YB-1. YB-1 interacts with amyloid-b fibrils. (A) The N-terminally located autoantibody epitope is mapped to the minimal binding sequence in the plasma and liquor of patients with Alzheimer's disease using an exceptionally fine peptide array. (B) That for the YB-1:b-amyloid interaction responsible YB-1 fragment should be determined using specific YB-1 protein deletion fragments. In addition, the interaction is examined in the presence of YB-1 antibodies and tested for inflammatory changes in the cell model.
|
Photos: by UMMD, Melitta Schubert/Sarah Kossmann