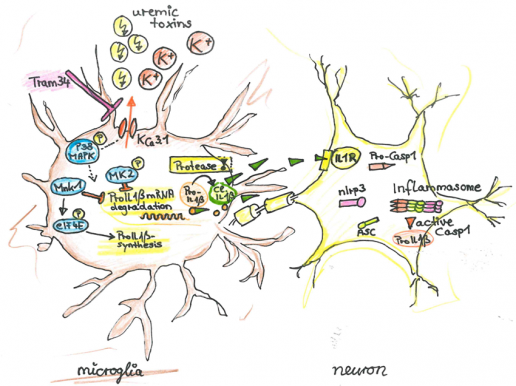
CS1: Uremia-induced microglial activation occurs via P-38-MAPK and Cathepsin C and mediates neuronal dysfunction via Il1β
|
|
Chronic kidney disease (CKD) causes cognitive impairment. We suspect that accumulating uremic toxins penetrate the impaired blood brain barrier in CKD.We hypothesize that CKD activates microglia contributing to local inflammasome activation. The Azo dye Evans Blue readily penetrates into the brain parenchyma in a mouse model of chronic kidney disease (5/6 nephrectomy, NX), but not in sham operated animals, indicating that uremia causes BBB disruption. Using ex vivo thallium-autometallography we demonstrate altered K+-metabolism in the CNS of mice with CKD. Comparison of NX C57BL/6 and NX NLRP3-/- mice revealed a comparable number of Tl+ positive activated microglia, indicating that the uremia-induced K+-dyshomeostasis in microglia is independent of the NLRP3 inflammasome. However, in NX NLRP3-/- mice neuronal Tl+ uptake was improved (compared to NX C57BL/6 controls), indicating that the NLRP3 inflammasome causes CKD-induced K+-dyshomeostasis of neurons. Using the KCa3.1-inhibitor TRAM-34 we demonstrate that K+-dyshomeostasis is required for microglia activation in vitro and in vivo. K+-dyshomeostasis is associated with impaired p38phosphorylation. The p38 pathway leads to the increased synthesis of pro-IL1β mRNA. The activation of microglia in CKD leads to Il1β secretion, cleaved by a Caspase8-RIPK1/3 alternative pathway. TRAM-34-treated mice improved in cognitive tests. We propose that activation of microglia independently of the canonical inflammasome is pivotal in the pathophysiology of CKD-induced cognitive decline. These results may lay ground for new therapeutic targets in CKD-associated dementia.
|
Photos: by UMMD, Melitta Schubert/Sarah Kossmann