AP5: Insight into the regulation of innate immune responses in Streptococcus pneumoniae-induced COPD exacerbation
|
|
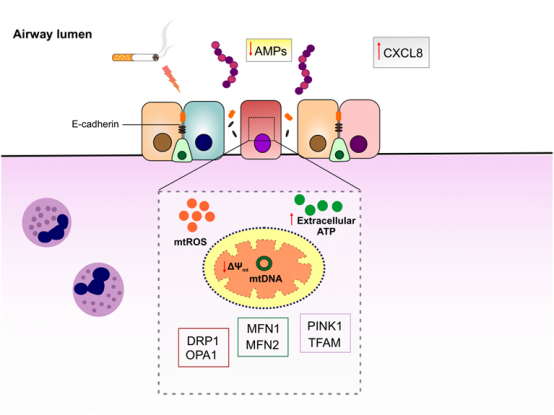
Chronic obstructive pulmonary diseases (COPD) is a heterogeneous respiratory disorder with huge economic and health burdens. Cigarette smoking is considered as the major risk factor for development of COPD, inducing sustained inflammatory response as well as decline in lung function. Acute worsening of COPD symptoms or COPD exacerbation is thought to be caused by bacterial and viral infections. Streptococcus pneumoniae (S. pneumoniae) is a third frequent bacteria isolated during COPD exacerbation that escalates condition by eliciting further oxidative stress and inflammation. Mitochondria are not only responsible for cellular bioenergetics but also recently found to be involved in innate immune responses to pathogens. Mitochondrial function is impaired in lung epithelial cells of COPD patients as well as smokers. However, the mechanistic pathway linking mitochondrial dysfunction to airway epithelial innate immune responses in COPD exacerbation is still lacking. In particular, host defense peptides/proteins (HDPs) constitute a robust innate immune defense restricting pathogens in epithelial luminal surface. Decreased production or activity of HDPs in airway epithelial cells may contribute to pathogenesis of COPD exacerbation. We hypothesize that mitochondrial dysfunction as a result of cigarette smoke exposure dampens innate immune response to S. pneumoniae in airway epithelium, which leads to decrease in HDPs, epithelial barrier disruption and as such further susceptibility to other pathogens. For this objective, we will be exploited state-of-the-art in vitro models of COPD exacerbation. First, mitochondrial dysfunction will be induced by submerging immortalized airway epithelial cells in cigarette smoke extract (CSE) followed by infecting with S. pneumoniae. To confirm induction of mitochondrial dysfunction, the protein levels of mitochondrial damage-sensitive proteins will be quantified. Furthermore, mitochondrial-specific reactive oxygen species (mtROS) and intracellular ROS levels, which are representative of oxidative stress status in the cells, will be measured. In order to characterize the role of mitochondrial dysfunction in airway epithelial innate immune responses, restoration of mitochondrial antioxidant activity by mitochondrial-targeted antioxidant MitoTEMPO will be tested. The impact of mitochondrial dysfunction on airway epithelial innate immune responses will be examined by quantifying the expression of neutrophil chemoattractant genes, the levels of cytokines and proteins involved in inflammatory pathways and host defense, as well as antibacterial activity of the epithelial cells. Changes in mitochondrial oxidative respiration activity will be determined by using Seahorse test (cooperation with Project 5). Furthermore, COPD exacerbation model will be induced by directly exposing well-differentiated primary airway epithelial cells from healthy and COPD subjects to CS and S. pneumoniae to fully recapitulate the condition (in collaboration with Prof. Pieter Hiemstra, LUMC, The Netherlands).
Schematic representation of proposed model of airway epithelial barrier disruption in COPD exacerbation. Cigarette smoke exposure induces mitochondrial dysfunction in airway epithelial cells that may in turn disrupt innate immune responses to pneumococci and increase the permeability of airway epithelial cells. |
Photos: by UMMD, Melitta Schubert/Sarah Kossmann