P1-1: Helicobacter pylori infection and gastric barrier function
Phatcharida JantareePhD Student
Michael NaumannProject Leader |
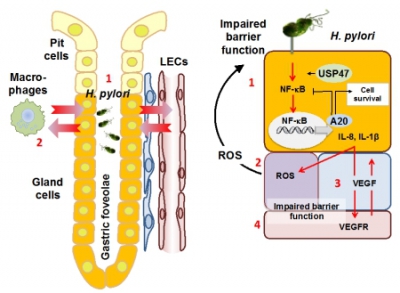
The development of a chronic inflammation in the stomach can be triggered by H. pylori, thus the human pathogen is a risk factor for gastric diseases including gastric cancer. The H. pylori-induced transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF‑kB) is involved in the pro-inflammatory response and cell survival in the gastric mucosa, and represents a trailblazer of gastric pathophysiology. H. pylori infection is not restricted to the epithelial cell populations, but due to cellular interactions within a specific disease-promoting micromilieu involve, e.g. subepithelial myofibroblasts. In order to identify suitable therapeutic targets and diagnostic markers for the prevention and treatment of chronic gastric diseases, we plan to identify intra- and intercellular signaling mechanisms promoting a maladaptive response at the epithelial gastric barrier. These studies will scrutinize the role of NF-κB (cooperation with Project 10 and Project 11) and deubiquitinylases, and modulation of protein-expression via the ubiquitin-proteasomal system. In systematic approaches we intend to study whole gastric tissue arrays from H. pylori infected patients (gastritis → adenoma → adenocarcinoma), aiming to identify DUBs as specific molecular pathologic biomarkers. The identified factors involved in H. pylori-associated chronic gastric diseases will be selected for detailed mechanistic studies using in vitro co-culture systems combining epithelial cells or gastric organoids, e.g. with myofibroblasts (cooperation with Associated Project 2, Associated Project 4 and Project 6). Drug design using biomolecular modeling/computational chemistry and preclinical efficacy tests will be conducted to evaluate the potential therapeutic value of potential novel targets.
H. pylori crosstalk with mucosal epithelial cells, fibroblasts etc. The molecular gastric pathology studies address the role of DUBs, e.g. A20, USP47 in H. pylori-induced NF-κB-dependent chemokine release and cell survival. The chronic inflammatory micromilieu and maladaptive processes will be studied in biopsies from patients. Explanted cells from patient material will be differentiated in pit- and gland-type organoids to perform knockdown of DUBs prior to infection. To investigate the crosstalk of epithelial gland-type cells with LEndoC, co-cultures will be analyzed in Boyden chamber and microfluidic devices. |
Photos: by UMMD, Melitta Schubert/Sarah Kossmann