P2-1: Impact of epigenetic imprinting on the perpetuation of intestinal inflammation and dysplasia
Elif Gelmez-BitterlichPhD Student
Dunja BruderProject Leader
|
To regain the homeostatic balance in chronically inflamed conditions the organism relies on regulatory mechanisms not observable under acute inflammatory conditions. On the molecular level these mechanisms are in part related to epigenetic gene regulation. Specific patterns of posttranslational histone modifications correlate with different gene activity states. The impact of posttranslational histone modification on maladaptive processes during chronic inflammatory conditions at the intestinal barrier remains unknown. To access inflammation induced histone modification in the intestinal epithelium at different disease states we will use the DSS colitis model to induce acute intestinal inflammation (group I) followed by convalescence (reestablishment of tissue homeostasis; group II), chronic intestinal inflammation (group III) as well as colorectal cancer (group IV, DSS treatment in combination with the carcinogen azoxymethane). Ex vivo isolated IEC will be subjected to histone acetylation and methylation analyses. Matching conventional transcription microarray analysis allows linking identified histone modifications to transcriptional alterations and cellular phenotypes (e.g. senescence, malignant transformation, cooperation with Project 1). Molecular signatures identified in the murine model will be validated in tissue biopsies obtained from human IBD and colorectal cancer patients (collaboration with Dr. Schulz, Clinic for Gastroenterology). To elucidate if inflammation-related molecular imprinting of IEC alters the micromilieu and immune cell composition in the gut emphasis will be put on the stage-specific characterization of secreted mediators (chemokines, cytokines, immunosuppressive mediators) as well as surface molecules involved in the direct interaction with intestinal immune cells (cooperation with Project 6 and Project 8). To evaluate the impact of IEC maladaptation on capillary function we will ascertain the endothelial phenotype (cellular senescence, expression of adhesion molecules, glycocalyx, cooperation with Project 1, Project 4, and Project 5). The mechanistic relevance of newly identified targets will be determined using genetic or pharmacological inhibitory approaches (cooperation with Project 8). These studies will uncover molecular and cellular maladaptive processes promoting the transition from acute to chronic intestinal inflammation and predisposing to tumor development.
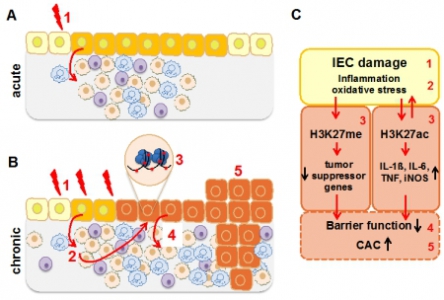
Epigenetic modification in iEpiC in the perturbation of intestinal inflammation and the development of colitis-associated cancer (CAC). A: Environmental triggers induce acute damage in iEpiC (1), resulting in transient immune activation and immune cell recruitment. In general, intestinal inflammation is self-limiting. B: In case of sustained epithelial damage (B), recruited inflammatory immune cells and their mediators result in exaggerated oxidative stress response (2) which can induce DNA damage and epigenetic alterations in iEpiC (3), resulting in perpetuation of inflammation, further epithelial barrier dysfunction (4) and eventually progression of chronic intestinal inflammation to colitis-associated cancer (CAC, 5). C: Schematic representation of the proposed disease mechanisms. |
Photos: by UMMD, Melitta Schubert/Sarah Kossmann