Akash Mathew

Akash Mathew
Former PhD Student
Project 7-1
P7-1: Maladaptive cross-talk at the glomerular filtration barrier
Akash MathewPhD Student Berend IsermannProject Leader |
Diabetic nephropathy (dNP) is the leading cause of renal failure in industrialized countries. Beyond inhibition of the angiotensin-system efficient therapies for dNP are lacking. While the relevance of individual cell components at the glomerular filtration barrier is well-studied, the mechanisms through which these cells coordinately regulate glomerular homeostasis and function remain largely unknown. Persistent hyperglycaemia promotes cellular dysfunction through maladaptive mitochondrial and ER-signalling. This is associated with altered PTMs of important homeostatic regulators (e.g. NF-κB, HIF, sXBP-1, HSPs). These maladaptive responses are molecular fixed and perpetuated through PTM’s and epigenetic reprogramming. We previously demonstrated that cellular interactions and protease-dependent signaling at the glomerular filtration barrier determine the highly specialized glomerular cell phenotype and epigenetically control expression of the redox-regulator p66Shc. These interactions are disrupted in a hyperglycaemic milieu. How cells at the glomerular filtration barrier interact during the maladaptive process, resulting in a specific disease promoting micromilieu, remains unknown. We will use an innovative co-culture system mimicking the glomerular filtration barrier to conduct kinetic studies aiming to identify extracellular mediators (e.g. coagulation proteases, YB-1, syndecan-4) and PTMs determining the cellular phenotype and function (cooperation with Project 6 and Project 8). This in vitro model will enable us to include tubular cells. These studies will be supplemented with expression profiling and ChIP sequencing (e.g. NF-κB, HIF, sXBP-1; cooperation with Project 6). Kinetic in vivo imaging analyses using genetically designed mice (loss or gain of function in the proteostasis network) will be used to ascertain the mechanistic relevance of identified regulators in vivo (cooperation with Project 4). Findings will be validated using in vitro (loss-/gain-of-function) and in vivo (tissue-specific gene-modulation, tubular-, podocyte-, or endothelial-specific Cre-expression) approaches as well as ex vivo analyses of human tissue biopsies.
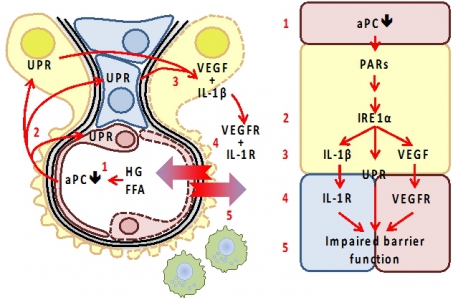
Maladaptive intercellular cross-talk at the GFB impairs barrier function. Elevated glucose (high glucose, HG) and free fatty acid (FFA) concentrations cause endothelial dysfunction, impairing EndoC-dependent protein C activation (1). Activated protein C (aPC) acts on endothelial cells (red), but also on podocytes (epithelial cells; yellow) and mesangial cells (blue), conveying cytoprotective effects via protease activated receptors. Loss aPC induces the unfolded protein response (UPR, 2) by directly or indirectly (via p66Shc) modulating ER-stress signaling (IRE1α). We speculate that IRE1α orchestrates UPR, inflammasome activity (IL-1β), and VEGF release (3). The corresponding mediators co-ordinately alter GFB function through receptor dependent mechanisms (4, 5). Excess IL-1β, VEGF, or yet to be identified mediators impair the barrier function. |
Photos: by UMMD, Melitta Schubert/Sarah Kossmann