Eliza von Gehlen

Eliza von Gehlen
Medical Doctor Project 11
MD11: Role of lipid receptor signaling in the differentiation and effector function of pathogenic Th17 cells in central nervous system autoimmunity
|
|
Multiple sclerosis (MS) is an autoimmune disease characterized by chronic inflammation, altered blood-brain barrier, demyelination, gliosis and neurodegeneration of the central nervous system (CNS). The pathogenesis of MS involves a complex interplay of genetic, environmental and immunological factors, including dysregulation of Th17 cells. In this project, we will investigate how the lipid metabolism of Th17 cells contributes to their pathogenic potential in mediating experimental autoimmune encephalomyelitis (EAE), an experimental model of MS. By combining metabolic analyses with single cell transcriptomics of spinal cord CD45+ leukocytes, we will elucidate how defective lipid signaling in Th17 cells modifies the blood-brain barrier and the complex interplay of innate and adaptive immune cells during central nervous system autoimmunity.
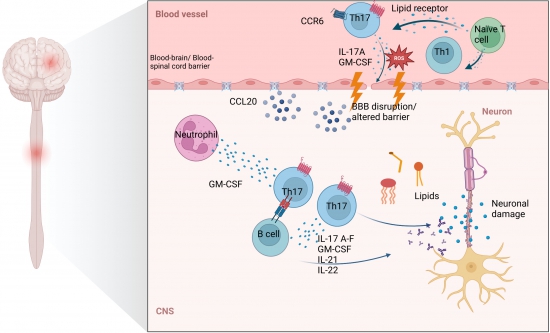
A central feature in the pathogenesis of multiple sclerosis is the activation of autoreactive T cells peripheral, notably Th17 and Th1 cells, who encounter specific antigens that mimic myelin proteins presented by antigen-presenting cells (APCs). These T cells migrate across the blood-brain barrier (BBB). CCR6 is a chemokine receptor expressed on Th17 cells and important for migration. It binds to CCL20 which is highly upregulated in the CNS during inflammation. The cytokines IL17-A-F, GM-CSF, IL-21, and IL-22 are produced by Th17 cells and are known to contribute to the modulation of tight junctions at the BBB, in example through the induction of oxidative stress in endothelial cells, which leads to increased permeability of the BBB facilitating the invasion of immune cells. In the CNS, autoreactive pathogenic Th17 cells are reactivated by local APCs such as microglia and dendritic cells and initiate an inflammatory response. Th17 cells recruit additional immune cells including CD8+ T cells, B cells, macrophages and neutrophils enhancing the production of other pro-inflammatory cytokines and chemokines and amplifying the inflammatory response, which finally results in neuronal damage. |
Photos: by UMMD, Melitta Schubert/Sarah Kossmann