Lorena Ferino

Lorena Ferino
PhD Student
Project 1-2
P1-2: USP48-dependent regulation of NF-kB and cell survival in the infected gastric mucosa
Lorena FerinoPhD Student
Michael NaumannProject Leader |
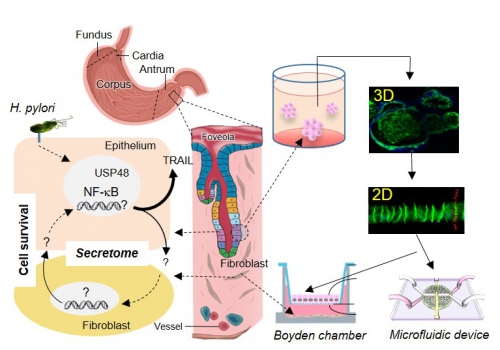
The human pathogen Helicobacter pylori the is a risk factor for gastric diseases including gastric cancer. H. pylori colonizes the gastric epithelium and the disease-promoting microenvironment involve, e.g. subepithelial fibroblasts. The H. pylori-induced transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF‑kB) is involved in the pro-inflammatory response and cell survival in the gastric mucosa, and represents a trailblazer of gastric pathophysiology. In order to identify suitable therapeutic targets and diagnostic markers for the prevention and treatment of chronic gastric diseases, we plan to identify intra- and intercellular signaling mechanisms promoting a maladaptive response at the epithelial gastric barrier. These studies will scrutinize the role of NF-κB (cooperation Project 11-2, Project 13-2 and MD 8) and deubiquitinylases (DUBs), and modulation of protein-expression via the ubiquitin-proteasome system. The impact of DUBs on maladaptive processes in the gastric mucosa (microenvironment) will be investigated by co-culture of gastric monolayer organoids and fibroblasts in the Boyden chamber and in microfluidic devices. Factors relevant for the maladaptation will be identified in the secretome and the metabolome of the cells and assessed in knockout cells (cooperation Project 12-2, Project 13-2 and Project 14-2). In addition, the expression of NF-kB and DUBs will be analyzed in whole gastric tissue arrays from patients with B-type gastritis and chronic gastritis.
H. pylori-induced cellular crosstalk in the gastric mucosa. Molecular pathological studies of the stomach address the role of deubiquitinylases (DUBs), e.g. USP48, in H. pylori-induced NF-kB activity and H. pylori-associated apoptotic cell death in the gastric mucosa. Maladaptive processes in the gastric microenvironment are investigated by co-culture of gastric monolayer (2D) mucosoids and fibroblasts in the Boyden chamber (static conditions) and in microfluidic devices (fluid flow conditions). Factors relevant for the maladaptation will be identified in the secretome and the metabolome of the cells and assessed in knockout cells. |
Photos: by UMMD, Melitta Schubert/Sarah Kossmann