Aaron Hoffmann

Aaron Hoffmann
PhD Student
Project 4-2
P4-2: Characterization of the specific functional relevance of perivascular mast cells in skin inflammation
|
|
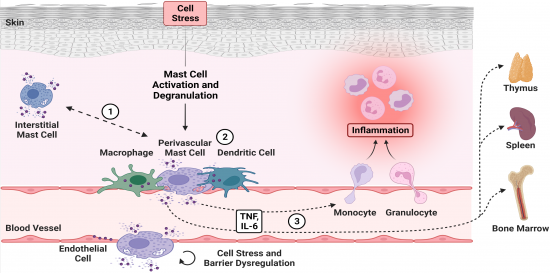
As the outermost layer of an organism, the skin is highly exposed to noxious environmental influences. By sensitizing with allergens or haptens able to penetrate this physiological barrier, an immunological response can be induced. In Europe, more than 20% of the population is affected by allergic skin diseases, such as atopic dermatitis or allergic contact dermatitis. These inflammatory skin disorders are a main cause for occupational disability and thereby a massive burden for the patients and the health care system. Recently, we could demonstrate that directional release of active mediators including tumor necrosis factor (TNF) by perivascular mast cells (MCs) into the blood stream initiates a vascular response and neutrophil infiltration. Moreover, this process affects immune cells systemically in distant organs, such as spleen and bone marrow. We hypothesize that repeated MC activation can lead to chronic disorders by a dysregulation of the vasculature or long-term systemic effects on myeloid immune cell populations. Given the longevity of tissue-resident MCs, their heterogeneity in both distinct tissues and within the skin, as well as their plasticity during inflammation is poorly understood. We therefore aim to characterize perivascular skin MCs and to investigate their specific functional relevance in skin inflammation, in particular in the activation and integrity of the endothelial barrier (cooperation with Project 6). Further, we will study their organ-spanning acute and long-term effects (cooperation with Project 2, Project 8, Project 11, Project 12). Since MCs are known to interact with other cells along the blood vessels, such as dendritic cells or macrophages, our objective is further to study the perivascular immune cell niche. To address our topics, we will utilize novel mouse lines with specific knockouts of surface receptors that reduce the alignment and directional intravascular degranulation of perivascular MCs. We will use in vivo approaches such as intravital 2-photon fluorescence microscopy to analyze the intercellular network along the vascular system, and cross-validate our findings with in vitro cell communication assays (cooperation with Project 6). We aim to ascertain the mechanistic effects of MC degranulation on tissue homeostasis, gene expression of transcription factors, cytokines and chemokines and hypoxia induced factors (cooperation with Project 2, Project 6, Project 8 and Project 12). These studies will thereby assess the specific features and functional relevance of perivascular MCs in acute and chronic skin inflammation in order to identify novel therapeutic targets.
Mast cells in the perivascular immune cell niche have critical functions during skin inflammation. We hypothesize that within the perivascular immune cell niche, MCs exhibit a distinct phenotype compared to non-vessel attached interstitial MCs (1). Furthermore, these specialized characteristics promote the intercellular communication with adjacent cells of the perivascular immune cell niche (2). Eventually, perivascular MCs might be involved in the transmission of local inflammation into systemic effects due to the directional release of MCGs into the bloodstream, even beyond the induction of immune cell recruitment (3). [Created by BioRender.com] |
Photos: by UMMD, Melitta Schubert/Sarah Kossmann