|

PhD Student

Project Leader
|
|
Renal tubular epithelial cells are amongst the most metabolically active cells in the human body and are therefore highly susceptible to injury (e.g. toxins, hypoxia). If the insults are sustained, they can lead to maladaptive responses, including cell death and a subsequent loss of barrier function, which ultimately result in reduced kidney function.
Previous studies in mouse models investigate the role of the cold shock proteins Y‑box binding protein-1 (YB-1) and DNA binding protein-A (DbpA) following acute kidney injury. Heterozygote YB-1 knockdown mice showed enhanced tubular damage following ischemia reperfusion (I/R); whereas damage was markedly reduced following tubular obstruction (UUO). Indicating that YB-1 can both prevent and promote renal injury. Furthermore, we recently showed that YB-1 coordinates the proximal tubular phenotypic activation and sodium-glucose cotransporter-2 expression following high salt diet (HSD) induction in mice. Our recent work demonstrates that a DbpA knockout protects mice from acute hypoxic kidney injury. Previous results also show the co-localization of DbpA within mitochondria, indicating its relevance with mitochondrial metabolic activity. Collectively, cold shock proteins determine tubular cell fate decisions in an injury-specific manner. Since the above-mentioned data is based on in vivo mouse models, the underlying molecular mechanisms remain unclear.
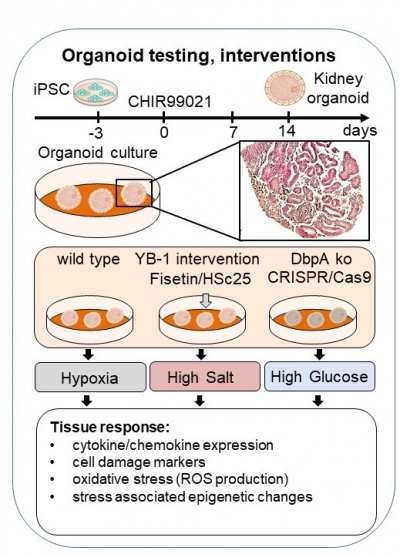
Based upon our initial data, we hypothesized that targeting DbpA in patients could be beneficial for the treatment of acute kidney injury. Therefore, the first step is to reproduce our data in a human system. For this, we will take advantage of our recently established organoid cultures, which reproduce many features of the architecture seen in an intact organ (kidney). Utilizing CRISPR/Cas technology, we will induce a knockout of DbpA in human cells in order to test whether this reproduces the protective effect we observe in mice under hypoxia. Furthermore, this model system will allow us to test the influence of environmental factors (high salt, high glucose, bacteria) on cold shock protein expression as well as their influence on the metabolic activity of the cells and the cellular responses to injury (e.g. cellular toxicity). This system is also suitable to test small molecule inhibitors to see whether we can reproduce the protective effects that are observed upon genetic deletion. Thus, this system brings us a step closer to developing new therapeutic strategies to treat kidney disease.
|