Neşe Güvençli

Neşe Güvençli
Former PhD Student
Project 13-2
P13-2: Endothelial cell-triggered metabolic maladaptation of CLL cells controls therapeutic resistance and immune escape
|
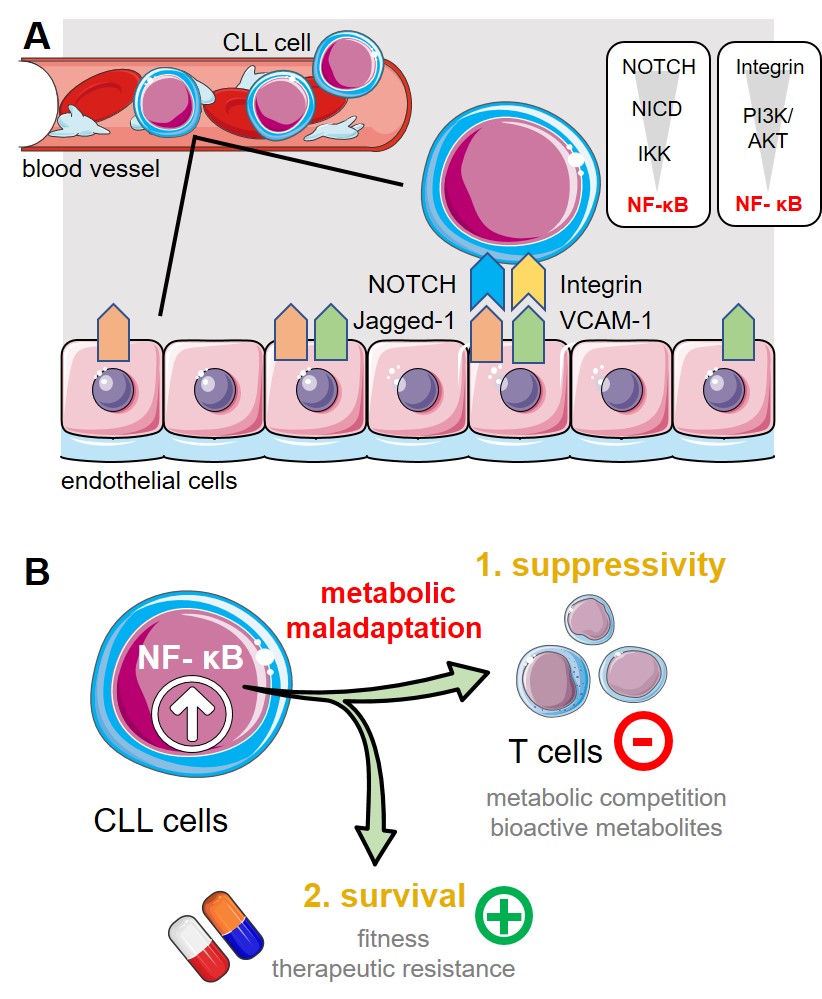
Chronic lymphocytic leukemia (CLL) represents the most common leukemia amongst adults in the Western world. Mature B-cell-derived cells progressively accumulate in the patients’ peripheral blood, lymphatic tissues, and bone marrow. Despite improved therapeutic approaches, allogeneic hematopoietic stem cell transplantation remains the only curative treatment option. One of the CLL cell’s hallmarks is their metabolic perturbation that affects both production and utilization of energy (i.e. bioenergetics) as well as redox homeostasis. We have previously shown that circulating CLL cells display increased oxidative phosphorylation (OXPHOS), mitochondrial biogenesis, and mitochondrial production of reactive oxygen species (ROS). Additionally, we described further CLL-related immune escape mechanisms that include the induction of myeloid derived suppressor cells (MDSCs) or an enhanced expression of the immune checkpoint molecule PD‑L1. Bidirectional interaction between CLL cells and the surrounding non-transformed stromal compartment (especially in the bone marrow) and extracellular matrix components extend CLL survival and protect from chemotherapeutics. We found this is at least partly mediated via bioenergetic reprogramming and formation of immune escape variants, both of which are of particular importance for drug sensitivity and immunotherapeutic concepts including among others monoclonal antibodies or genetically engineered CAR T-cells. Endothelial cells (ECs) also represent a component of the tumor stroma and previous data suggest an involvement in CLL pathobiology. Similarly to the bone marrow stroma, ECs express several surface markers that may allow cell-to-cell contact between them and CLL cells (e.g. NOTCH and integrin ligands) all culminating at NF-κB. Hence, we hypothesize that a chronic bidirectional crosstalk between ECs and CLL cells leads to metabolic maladaptation in CLL cells. First, we will analyze the metabolic phenotype and the underlying signaling pathways of human/murine CLL cells in response to their interaction with ECs. We will employ different methods for metabolic profiling using an in vitro co-culture model (cooperation with Project 2) including single-cell RNA sequencing (cooperation with Project 12), targeted metabolomics, and flow cytometry-based analyses of metabolic markers/parameters as well as intracellular signaling components (focus on NF-κB signaling in cooperation with Project 1). As the next step, we will delineate the impact of EC-mediated metabolic alterations on the CLL cells’ drug resistance and their ability to hamper T cell immunity by challenging the CLL cells with conventional agents and targeted approaches and analyzing T-cell activation, function, and exhaustion. To evaluate the influence of EC-mediated metabolic maladaptation of CLL cells on those processes, we will inhibit the respective metabolic pathways as well as genetically ablate involved metabolic proteins/enzymes via CRISPR/Cas9 approaches. Last, we will interfere with the identified (metabolic) mechanisms in vivo using a preclinical model and evaluate the impact on the CLL cell metabolism, T cell compartment, and response to therapies. Proposed model of interaction between ECs and CLL cells. (A.) CLL cells display a recurrent and often yearlong contact to the epithelial barrier during circulation and/or transendothelial migration into e.g., the bone marrow or spleen. ECs express amongst other cell surface proteins the NOTCH-ligand Jagged-1 and integrin ligand VCAM-1. Triggering NOTCH (by Jagged-1) or integrin a4b1 (by VCAM-1) leads both to activation of NF-kB in CLL cells. High NF-kB activity represents one of the CLL cells’ hallmarks. (B.) NF-kB signaling can control cellular bioenergetics. A sustained NF-kB pathway activation (as elicited by ECs) could promote metabolic maladaptation in CLL cells. Thereby, it could (1.) enhance the CLL cells’ ability to suppress immune responses by e.g., substrate depletion and/or production of bioactive metabolites and it could (2.) promote the malignant cells’ survivability especially in presence of chemotherapeutics. Abbreviations: NICD; Notch intracellular domain, IKK; IkB kinase, PI3K, phosphoinositide 3-kinase. |
Photos: by UMMD, Melitta Schubert/Sarah Kossmann