Martin Voss

Dr. Martin Voss
Former PhD Student
Project 4-1
P4-1: Relevance of mast cells in maladaptation of the epidermal and endothelial barrier during chronic skin inflammation
Martin VossPhD Student
Anne DudeckProject Leader |
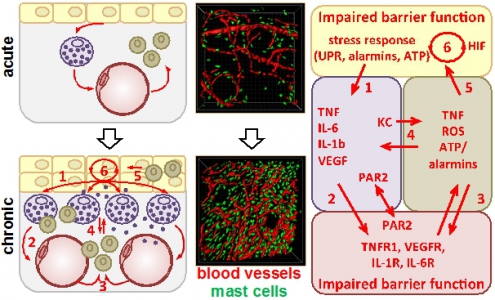
Contact allergy is an inflammatory skin disease of high socioeconomic relevance. We recently demonstrated an intimate interaction of dermal endothelial cells and mast-cells (MCs) in the skin. Importantly, MCs initiate the vascular response to haptens and neutrophil infiltration, and amplify the T cell-driven adaptive immunity. Surprisingly, we observed a massive MC hyperplasia several weeks after hapten-challenge, predisposing to chronified skin inflammation. Based on these observations we hypothesize that the perivascular MC hyperplasia provokes a disease-promoting micromilieu resulting in a permanent dysregulation of endothelial barriers. By combining longitudinal intravital imaging with cell biology approaches we aim to quantify parameters of vascular integrity, endothelial cell activation and luminal glycocalyx constitution. The interaction of the glycocalyx with the extracellular proteome will be analyzed (cooperation with Project 7). To assess the effect of perpetuated inflammation on entire skin vascularity, we will analyze the vessel structure and 3D architecture. To directly assess the causality of MCs for epidermal and vascular alterations in perpetuated skin inflammation we will utilize novel mouse models of constitutive or inducible MC-depletion. To characterize the disease-promoting micromilieu at the pathophysiological endothelial barrier we need to identify the cellular actuators and regulators beyond MCs and therefore aim to analyze the impact of endothelial cell activation and luminal glycocalyx on macrophage differentiation (cooperation with Project 6). The homeostasis of intercellular networks is tightly regulated by a number of tissue factors. We therefore aim to analyze alterations in the gene expression of transcription factors (cooperation with Project 9), cytokines and chemokines, complement and coagulation factors (cooperation with Project 5 and Project 6), hypoxia induced factors (cooperation with Project 2) and others, to identify key factors disrupting the endothelial barrier in the chronically inflamed skin as potential therapeutic targets. Maladaptive cell accumulation and intercellular communication lead to chronic skin inflammation. Acute hapten-induced skin inflammation (arrow) leads to an accumulation of mast cells (MC, violet) beneath the epidermal (yellow) barrier. Repeated hapten encounter in hapten-primed skin causes an exaggerated inflammation and neutrophil (green) infiltration potentially due to persistent MC degranulation that may culminate in structural and functional maladaptation of the endothelium (red) and epidermis. We hypothesize that the epidermal stress response (1) is causative for the accumulation of MCs which subsequently provoke endothelial activation (2) and neutrophil infiltration (3) via degranulation, cytokine/chemokine release and PAR2 activation. The exaggerated neutrophil influx vice versa results in persistent MC activation (4), perpetuated epidermal stress (5), and hypoxia (6) that finally culminate in endothelial dysfunction. |
Photos: by UMMD, Melitta Schubert/Sarah Kossmann