CS2: The role of VAMP3-IKKg/NEMO interaction in inflammation of atherosclerosis
|
|
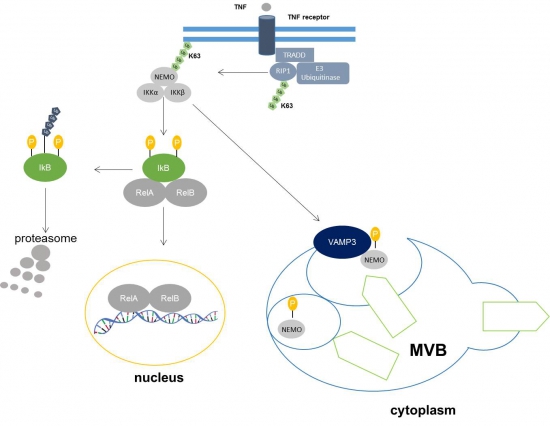
Atherosclerosis is the cause of vascular diseases like myocardial infarction and stroke. Cardiovascular diseases are the leading cause of mortality worldwide. Atherosclerosis comprises an inflammatory micromilieu which results in an impaired barrier function. This triggers the retention of Apolipoprotein B (apoB), which provokes an entry of monocytes and their differentiation into macrophages. One of the key steps in early atherosclerotic inflammation is the activation of NF-kappaB (NF-κB) signaling pathway in endothelial cells and macrophages. Essential for activation of the NF-κB signaling pathway is the IKK complex, which phosphorylates the inhibitor of NF-κB transcription factor, IΚB. Subsequently, IKB is proteasomally degraded allowing the NF-κB transcription factor to translocate into the nucleus. The IKK complex consists of IKKα and IKKβ as catalytical subunits, and the NF-κB essential modifier (IKKγ/NEMO). VAMP3 is a SNARE (soluble N-ethylmaleimide-sensitive-factor-attachment receptor) protein involved e.g. in endocytic and exocytic pathways. It is necessary for the fusion between autophagosomes and MVBs (Multivesicular Bodies) and interacts with NEMO. Hitherto we lack insights how the interaction of VAMP3 and IKKγ/NEMO modulates NF-κB signaling.
|
Photos: by UMMD, Melitta Schubert/Sarah Kossmann